How does soap keep you clean? A chemist explains the science of soap
- Soap has been used for over 5,000 years to clean bodies and clothes, dating back to ancient Babylon.
- The science behind soap lies in its unique molecular structure, which is both water-loving (hydrophilic) and water-fearing (hydrophobic), allowing it to effectively remove dirt, grease, and grime from skin.
- When you wash your hands with soap and water, the soap molecules form a micelle structure that traps the dirt and microorganisms on your skin, allowing running water to wash them away.
- Soap is not just effective against dirt, but also against microorganisms like bacteria, viruses, and fungi that can cause illness and disease, helping to keep billions of people healthy for thousands of years.
- Washing your hands with soap and water for at least 20 seconds is crucial in removing germs and keeping you clean, making it an essential part of daily hygiene.

Curious Kids is a series for children of all ages. If you have a question you’d like an expert to answer, send it to CuriousKidsUS@theconversation.com.
How does soap clean our bodies? – Charlie H., age 8, Stamford, Connecticut
Thousands of years ago, our ancestors discovered something that would clean their bodies and clothes. As the story goes, fat from someone’s meal fell into the leftover ashes of a fire. They were astonished to discover that the blending of fat and ashes formed a material that cleaned things. At the time, it must have seemed like magic.
That’s the legend, anyway. However it happened, the discovery of soap dates back approximately 5,000 years, to the ancient city of Babylon in what was southern Mesopotamia – today, the country of Iraq.
As the centuries passed, people around the world began to use soap to clean the things that got dirty. During the 1600s, soap was a common item in the American colonies, often made at home. In 1791, Nicholas Leblanc, a French chemist, patented the first soapmaking process. Today, the world spends about US$50 billion every year on bath, kitchen and laundry soap.
But although billions of people use soap every day, most of us don’t know how it works. As a professor of chemistry, I can explain the science of soap – and why you should listen to your mom when she tells you to wash up.
The chemistry of clean
Water – scientific name: dihydrogen monoxide – is composed of two hydrogen atoms and one oxygen atom. That molecule is required for all life on our planet.
Chemists categorize other molecules that are attracted to water as hydrophilic, which means water-loving. Hydrophilic molecules can dissolve in water.
So if you were to wash your hands under a running faucet without using soap, you’d probably get rid of lots of whatever hydrophilic bits are stuck to your skin.
But there is another category of molecules that chemists call hydrophobic, which means water-fearing. Hydrophobic molecules do not dissolve in water.
Oil is an example of something that’s hydrophobic. You probably know from experience that oil and water just don’t mix. Picture shaking up a jar of vinaigrette salad dressing – the oil and the other watery ingredients never stay mixed.
So just swishing your hands through water isn’t going to get rid of water-fearing molecules such as oil or grease.
Here’s where soap comes in to save the day.
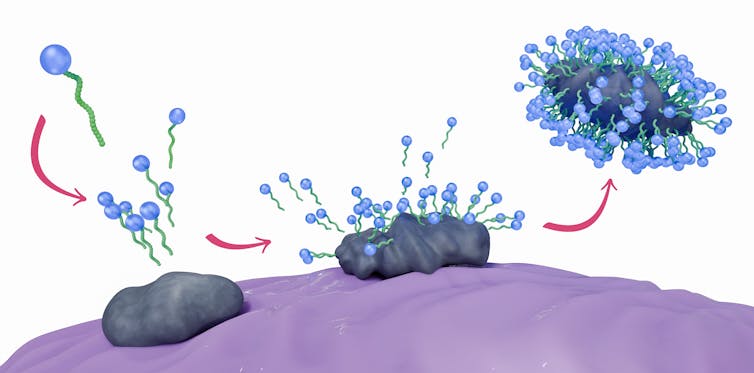
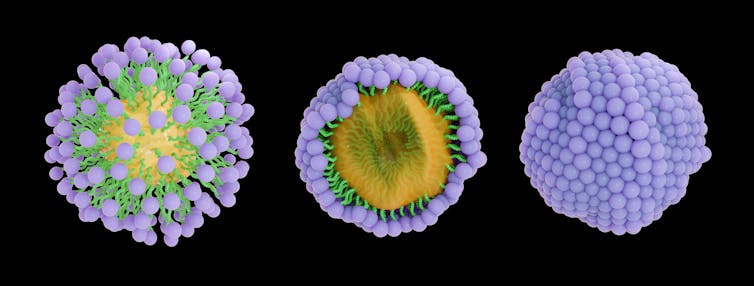
Soap, a complex molecule, is both water-loving and water-fearing. Shaped like a tadpole, the soap molecule has a round head and long tail; the head is hydrophilic, and the tail is hydrophobic. This quality is one of the reasons soap is slippery.
It’s also what gives soap its cleaning superpower.
Tumeggy/Science Photo Library via Getty Images
A microscopic view
To see what happens when you wash your hands with soap and water, let’s zoom in.
Picture all the gunk that you touch during the day and that builds up on your skin to make your hands dirty. Maybe there are smears of food, mud from outside, or even sweat and oils from your own skin.
All of that material is either water-loving or water-fearing on the molecular level. Dirt is a jumbled mess of both. Dust and dead skin cells are hydrophilic; naturally occurring oils are hydrophobic; and environmental debris can be either.
If you use only water to clean your hands, plenty will be left behind because you’d only remove the water-loving bits that dissolve in water.
But when you add a bit of soap, it’s a different story, thanks to its simultaneously water-loving and water-fearing properties.
TUMEGGY/Science Photo Library via Getty Images
Soap molecules come together and surround the grime on your hands, forming what’s known as a micelle structure. On a molecular level, it looks almost like a bubble encasing the hydrophobic bit of debris. The water-loving heads of the soap molecules are on the surface, with the water-fearing tails inside the micelle. This structure traps the dirt, and running water washes it all away.
To get the full effect, wash your hands at the sink for at least 20 seconds. Rubbing your hands together helps force the soap molecules into whatever dirt is there to break it up and envelope it.
It’s not just dirt
Along with dirt, your body is covered by microorganisms – bacteria, viruses and fungi. Most are harmless and some even protect you from getting sick. But some microorganisms, known as pathogens, can cause illness and disease.

velvelvel/iStock via Getty Images Plus
They can also cause you to smell if you haven’t taken a bath in a while. These bacteria break down organic molecules and release stinky fumes.
Although microorganisms are protected by a barrier – it’s called a membrane – soap and water can disrupt the membrane, causing the microorganism to burst open. The water then washes the remains of the microorganism away, along with the stink.
To say that soap changed the course of civilization is an understatement. For thousands of years, it’s helped keep billions of people healthy. Think of that the next time Mom or Dad asks you to wash up – which will likely be sometime soon.
Hello, curious kids! Do you have a question you’d like an expert to answer? Ask an adult to send your question to CuriousKidsUS@theconversation.com. Please tell us your name, age and the city where you live.
And since curiosity has no age limit – adults, let us know what you’re wondering, too. We won’t be able to answer every question, but we will do our best.
![]()
Paul E. Richardson receives funding from the NIH and NSF.
